Study Design
- Primary endpoint: Overall survival among all patients with newly diagnosed high-grade glioma, the primary outcome measure, was prolonged in the Gliadel arm.
- Exclusion criteria: Prior cytoreductive therapy or radiotherapy to the brain, multifocal disease, or clinically significant laboratory abnormalities.
- Patients were followed for at least 3 years or until death.
- For all patients except those with anaplastic oligodendroglioma, systemic chemotherapy was not allowed until recurrence.
- This study was the basis for the second FDA-approved indication of GLIADEL Wafer1
A Double-blind, Placebo-controlled, Randomized Trial In Newly-Diagnosed HGG Patients1,2,3
- References
- GLIADEL® WAFER (carmustine implant) for intracranial use [Prescribing Information]. Atlanta, GA: Arbor Pharmaceuticals, LLC; 2018.
- Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79-88.
- Westphal M, Ram Z, Riddle V, Hilt D, Bortey E; on behalf of the Executive Committee of the Gliadel® Study Group. Gliadel® wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir. 2006;148:269-275.
- Efficacy
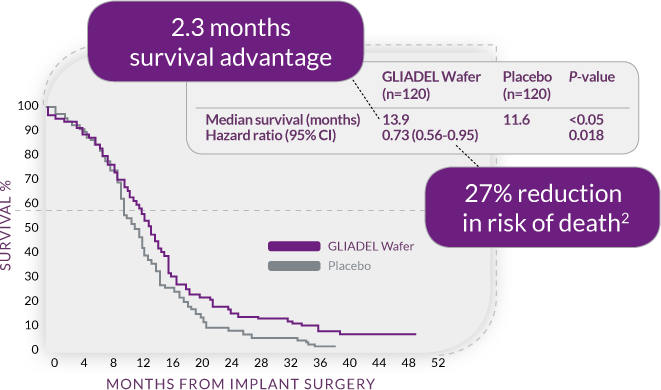
Survival Was Extended in Newly-Diagnosed HGG Patients
The use of GLIADEL Wafer during initial surgery resulted in1,2:
• 2.3 month survival advantage (P<0.05)
• 27% reduction in risk of death (HR=0.73, P=0.02; 95% CI, 0.56-0.95)
Overall Survival for HGG Patients Undergoing Initial Surgery1
REFERENCES
- GLIADEL® Wafer (carmustine implant) for intracranial use [Prescribing Information]. Atlanta, GA: Arbor Pharmaceuticals, LLC; August 2018.
- Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir. 2006;148(3):269-275.
- Safety
Deliver localized chemotherapy while limiting systemic exposure1,2
Safety profile for newly-diagnosed HGG patients
Per-Patient Incidence of Adverse Reactions
(Between Arm Differences of ≥4%)1- BODY SYSTEM
- GLIADEL® WaferN=120
- PlaceboN=120
- %
- %
- Gastrointestinal Disorders
- Nausea
- 22
- 17
- Vomiting
- 21
- 16
- Constipation
- 19
- 12
- Abdominal pain
- 8
- 2
- General Disorders and Administration Site Condition
- Asthenia
- 22
- 15
- Chest Pain
- 5
- 0
- Injury, Poisoning, and Procedural Complications
- Wound healing abnormalities*
- 16
- 12
- Musculoskeletal and Connective Tissue Disorders
- Back pain
- 7
- 3
- Psychiatric Disorders
- Depression
- 16
- 10
*Included (1) Fluid, CDS, or subdural fluid collection; (2) CSF leak; (3) Wound dehisence, breakdown, or poor healing; and (4) Subgaleal or wound effusions (including yellow discharge at the incision)
REFERENCES
- GLIADEL® Wafer (carmustine implant) for intracranial use [Prescribing Information]. Atlanta, GA: Arbor Pharmaceuticals, LLC; 2018.
- Engelhard HH. The role of interstitial BCNU chemotherapy in the treatment of malignant glioma. Surg Neurol. 2000;53:458-464.
Deliver chemotherapy DIRECTLY TO THE TUMOR BED1
Localized safety profile for newly-diagnosed HGG
Incidence of Local Adverse Reactionsa,1
- LOCAL ADVERSE REACTIONS
- GLIADEL® WaferN=120
- PlaceboN=120
- %
- %
- Cerebral edema
- 23
- 19
- Intracranial hypertension
- 9
- 2
- Cerebral hemorrhage
- 6
- 4
- Brain abscess
- 6
- 4
- Brain cyst
- 2
- 3
aNot seen at baseline or worsened if present at baseline.
REFERENCES
- GLIADEL® Wafer (carmustine implant) for intracranial use [Prescribing Information]. Atlanta, GA: Arbor Pharmaceuticals, LLC; 2018.

