Study Design
- Primary endpoint: overall survival from the time of polymer implantation.
- The follow-up period was up to 71 months.
- Patients included in this study were eligible for systemic chemotherapy.
- Additional eligibility criteria included: completion of external beam radiation therapy; no nitrosureas for 6 weeks and no other systemic chemotherapy for 4 weeks prior to enrollment.
- This study was the basis for the first FDA-approved indication of GLIADEL Wafer.1
A Double-blind, Placebo-controlled, Randomized Trial In Patients with Recurrent GBM1,2
- References
- GLIADEL® Wafer (carmustine implant) for intracranial use [Prescribing Information]. Atlanta, GA: Arbor Pharmaceuticals, LLC; 2018.
- Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345(8956):1008-1012.
- Efficacy
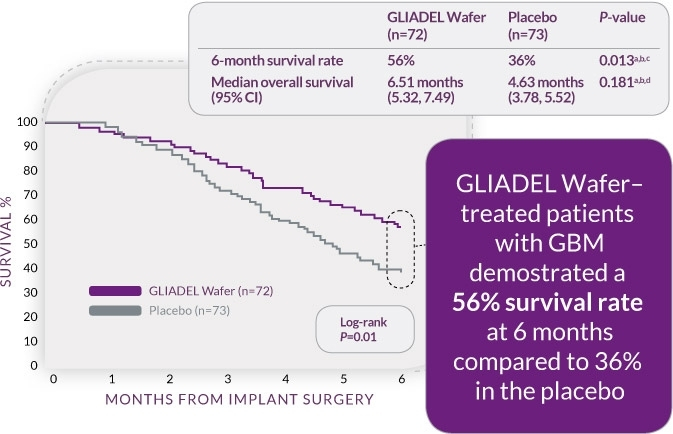
20% MORE PATIENTS SURVIVED AT 6-months with gliadel wafer.1
Median survival of GBM patients (n=145) increased by 41% from 18.4 weeks (4.6 months) with placebo to 25.9 weeks (6.5 months) with GLIADEL Wafer treatment
6-Month Survival for Patients Undergoing Surgery for Recurrent GBM1
- P-value not adjusted for multiple comparisons.
- Log-rank P-value.
- P-value by Gehan's generalized Wilcoxon Test was 0.015.
- P-value by Gehan’s generalized Wilcoxon Test was 0.021.
REFERENCES
- GLIADEL® Wafer (carmustine implant) for intracranial use [Prescribing Information]. Atlanta, GA: Arbor Pharmaceuticals, LLC; August 2018.
- Safety
Safety Profile in Recurrent GBM Patients
Per-Patient Incidence of Adverse Reactions (Between Arm Differences of ≥4%)1
- ADVERSE REACTION
- GLIADEL® WaferN=110
- PlaceboN=112
- %
- %
- General
- Fever
- 12
- 8
- Infectious
- Urinary tract infections
- 21
- 17
- Injury, Poisoning and Procedural Complications
- Wound healing abnormalities*
- 14
- 5
*Included (1) Fluid, CDS, or subdural fluid collection; (2) CSF leak; (3) Wound dehisence, breakdown, or poor healing; and (4) Subgaleal or wound effusions (including yellow discharge at the incision)
SAFETY PROFILE IN RECURRENT GBM PATIENTS
Incidence of Seizures, Hydrocephalus, and Cerebral Edema1
- ADVERSE REACTION
- GLIADEL® WaferN=110
- PlaceboN=112
- Patients with Seizures (%)
- Any seizures after wafer implantation
- 37
- 29
- New or worsening seizures
- 20
- 20
- Time to new or worsening seizures (days)a
- Mean (SD)
- 26.09 (0.75)
- 62.36 (48.66)
- Median
- 3.5
- 61.0
- %
- %
- Hydrocephalusb
- 5
- 2
- Cerebral edemab
- 4
- 1
aDays are from implantation to onset of first new or worsening seizure.
bNot seen at baseline or worsened if present at baseline.REFERENCES
- GLIADEL® Wafer (carmustine implant) for intracranial use [Prescribing Information]. Atlanta, GA: Arbor Pharmaceuticals, LLC; 2018.

